Functional and molecular characterization of a peptide transporter in the rat PC12 neuroendocrine cell line.
I Hussain, T Zanic-Grubisic, Y Kudo, C A Boyd
Index: FEBS Lett. 508(3) , 350-4, (2001)
Full Text: HTML
Abstract
We have studied functional properties of peptide transport in the pheochromocytoma neuroendocrine cell line from rat. The neutral peptide D-Phe-L-Ala (resistant to hydrolysis) is a good substrate for uptake into these cells. Transport is substantially inhibited by diethylpyrocarbonate pretreatment and is stimulated by external acidification. It is sodium-independent and, unexpectedly, insensitive to membrane potential. Peptide uptake is inhibited by a wide variety of other di- and tripeptides but not by amino acids. The neuropeptide kyotorphin (opioid dipeptide (L-Tyr-L-Arg)) inhibits uptake of labelled peptide and trans-stimulates efflux showing that it is a transported substrate. These findings are discussed in relation to the molecular basis and physiological role of this transport system.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
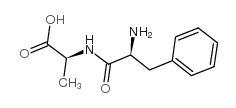 |
Phe-Ala
CAS:3918-87-4 |
C12H16N2O3 |
|
Alkali metal complexes of the dipeptides PheAla and AlaPhe: ...
2008-03-14 [ChemPhysChem 9 , 579-589, (2008)] |
|
Single-conformation ultraviolet and infrared spectroscopy of...
2008-04-09 [J. Am. Chem. Soc. 130(14) , 4795-807, (2008)] |
|
A novel inhibitor of the mammalian peptide transporter PEPT1...
2001-04-10 [Biochemistry 40(14) , 4454-8, (2001)] |
|
P3 cap modified Phe*-Ala series BACE inhibitors.
2004-01-05 [Bioorg. Med. Chem. Lett. 14(1) , 245-50, (2004)] |
|
Dipeptide transport characteristics of the apical membrane o...
1995-08-01 [Am. J. Physiol. 269(2 Pt 1) , L137-43, (1995)] |