| Structure | Name/CAS No. | Articles |
|---|---|---|
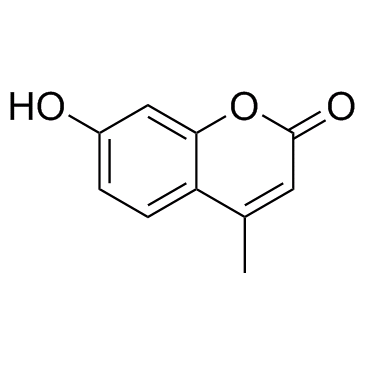 |
4-Methylumbelliferone
CAS:90-33-5 |
|
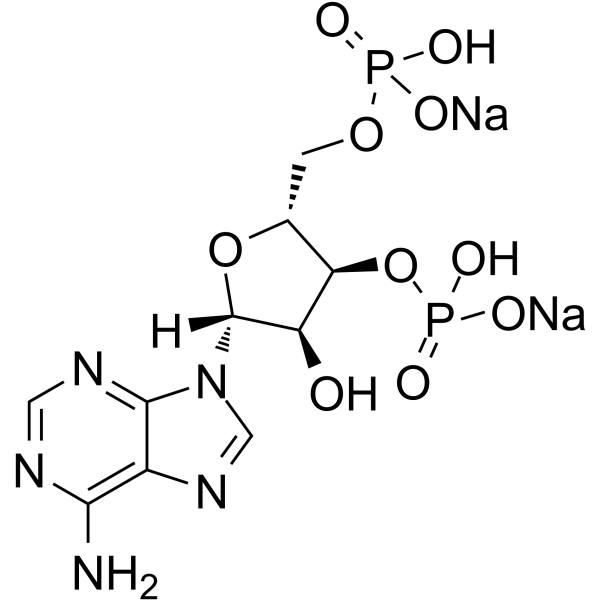 |
Adenosine 3',5'-diphosphate (sodium salt)
CAS:75431-54-8 |