Using chemical reactivity to provide insights into environmental transformations of priority organic substances: the Fe⁰-mediated reduction of Acid Blue 129.
Salma Shirin, Vimal K Balakrishnan
Index: Environ. Sci. Technol. 45(24) , 10369-77, (2011)
Full Text: HTML
Abstract
Sulfonated anthracenedione dyes are medium priority organic compounds targeted for environmental assessment under the Government of Canada's Chemical Management Plan (CMP). Since organic compounds undergo transformations in environmental matrices, understanding these transformations is critical for a proper assessment of their environmental fate. In the current study, we used zero-valent iron (ZVI) to provide insight into reductive transformation processes available to the anthracenedione dye, Acid Blue 129 (AB 129), a dye which is used in the textile industry. At high temperatures, we found that AB 129 was rapidly reduced (within 3 h) after being adsorbed onto the ZVI-surface, whereupon decomposition took place via multiple competitive and consecutive reaction pathways. Reaction products were identified using state-of-the-art accurate mass Liquid Chromatography-Quadrupole Time of Flight-Mass Spectroscopy (LC-QToF-MS). Five transformation products were identified, including a genotoxic (and thus, potentially carcinogenic) end-product, 2,4,6-trimethylaniline. The same products were found at room temperature, demonstrating that the transformation pathways revealed here could plausibly arise from biological and/or environmental reductions of AB 129. Our results demonstrate the importance of identifying reaction product arising from priority substances as part of the environmental risk assessment process.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
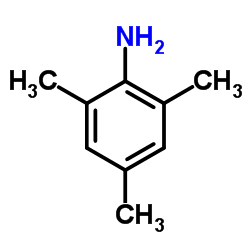 |
2,4,6-Trimethylaniline
CAS:88-05-1 |
C9H13N |
|
Stability of erythrocyte membrane and overall hemostasis pot...
2015-12-01 [Pharmacol. Rep. 67 , 1230-9, (2015)] |
|
Assessment of aniline derivatives-induced DNA damage in the ...
1999-01-01 [Teratog. Carcinog. Mutagen. 19(5) , 323-7, (1999)] |
|
Assessment of aniline derivatives-induced DNA damage in the ...
1999-12-01 [Cancer Lett. 147(1-2) , 1-4, (1999)] |
|
Effect of the location of hydrogen abstraction on the fragme...
2003-06-01 [J. Am. Soc. Mass Spectrom. 14(6) , 658-70, (2003)] |
|
2,4,5- and 2,4,6-Trimethylaniline and their hydrochlorides.
1982-04-01 [IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 27 , 177-88, (1982)] |