Silica-supported polyphosphoric acid in the synthesis of 4-substituted tetrahydroisoquinoline derivatives.
Stanimir Manolov, Stoyanka Nikolova, Iliyan Ivanov
Index: Molecules 18(2) , 1869-80, (2013)
Full Text: HTML
Abstract
We report herein an application of an α-amidoalkylation reaction, as an alternative efficient synthesis of 4-aryl- and 4-methyl-1,2,3,4-tetrahydroisoquinoline derivatives. The amides required for this purpose would result from reaction of aminoacetaldehyde dimethylacetal with different substituted benzenes in polyphosphoric acid, followed by acylation of the obtained amines with different acid chlorides or sulfochlorides. We compared the cyclisation step using conventional (milieu of acetic-trifluoracetic acid = 4:1) and solid supported reagents (SiO₂/PPA), as recovered, regenerated and reused without loss of its activity catalyst. We found that in comparison to conventional methods, the yields of the reaction are greater and the reaction time is shorter.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
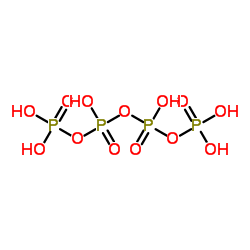 |
Tetraphosphoric acid
CAS:8017-16-1 |
(H)n+2.(P)n.(O)3n+1 |
|
Polyphosphoric acid trimethylsilyl ester promoted intramolec...
2003-10-30 [Org. Lett. 5(22) , 4061-4, (2003)] |
|
Synthesis and antitubercular evaluation of amidoalkyl dibenz...
2011-07-15 [Bioorg. Med. Chem. Lett. 21 , 4316-9, (2011)] |
|
Synthesis and insect antifeedant activity of aurones against...
2007-02-07 [J. Agric. Food Chem. 55(3) , 700-5, (2007)] |
|
Cellular formation of DNA and RNA and the relationship to tu...
2004-01-01 [Med. Hypotheses 62(1) , 97-111, (2004)] |
|
The MPEG monophosphate ester: synthesis and characterization...
2009-01-01 [J. Biomater. Sci. Polym. Ed. 20(14) , 2103-16, (2009)] |