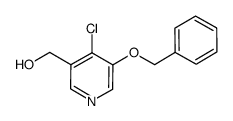
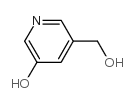
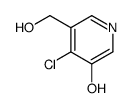
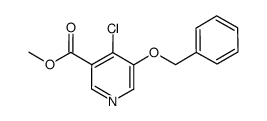
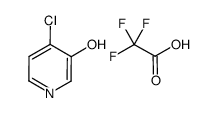
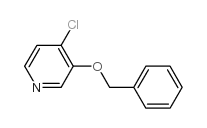
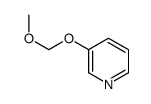
|
~8% |
|
~87% |
|
~55% |
|
~84% |