Determination of pipamperone in human plasma by high performance liquid chromatography.
I Luhmann, S C Szathmary, I Grünert
Index: Arzneimittelforschung 42(9) , 1069-72, (1992)
Full Text: HTML
Abstract
A sensitive and reproducible high performance liquid chromatographic method was developed for the determination of pipamperone (1'-[4-(4-fluorophenyl)-4-oxobutyl]-[1,4'-bipiperidine]-4'- carboxamide, Dipiperon, CAS 1893-33-0) in plasma samples. Pipamperone and its internal standard (piritramide or 1'-(3-cyano-3,3-diphenylpropyl)-[1,4'-bipiperidine]-4'-carbo xamide) were extracted from alkalinized plasma by liquid-liquid extraction and analysed on a reversed-phase (C18) column. In order to reduce analysis time, a gradient elution technique was applied. Chromatographic peaks were quantified by UV detection at two wavelengths. The method has a detection limit of 2 ng/ml, it proved to have good accuracy and precision, and was linear in the range of 2 to 400 ng/ml. The assay was extensively applied to study the pharmacokinetics in man after oral administration of the drug.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
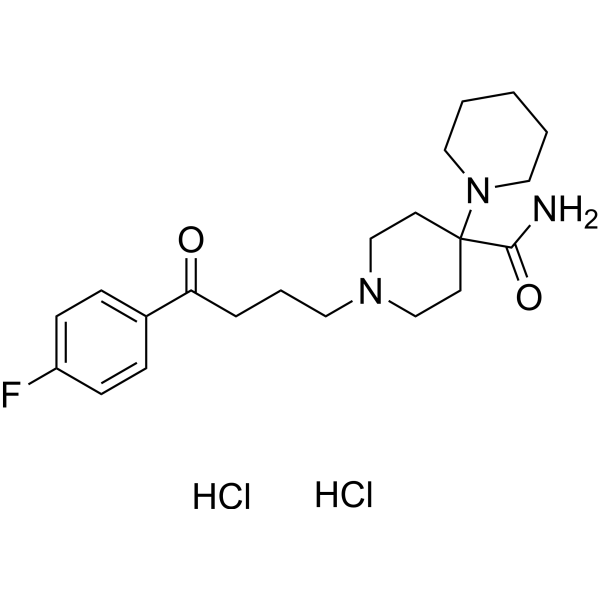 |
Pipamperone Dihydrochloride
CAS:2448-68-2 |
C21H32Cl2FN3O2 |
|
The use of dried blood spots for quantification of 15 antips...
2015-06-01 [Drug Test. Anal. 7 , 502-11, (2015)] |
|
Determination of Common Antipsychotics in Quantisal-Collecte...
2016-02-01 [Ther. Drug Monit. 38 , 87-97, (2016)] |
|
German insurer finds a third of people over 65 take five or ...
2013-01-01 [BMJ 346 , f3905, (2013)] |
|
Phelan-McDermid syndrome: clinical report of a 70-year-old w...
2013-01-01 [Am. J. Med. Genet. A 161A(1) , 158-61, (2013)] |
|
Evaluation of serotonin-2A receptor occupancy with 123I-5-I-...
2008-08-01 [Nucl. Med. Commun. 29(8) , 724-9, (2008)] |