| Structure | Name/CAS No. | Articles |
|---|---|---|
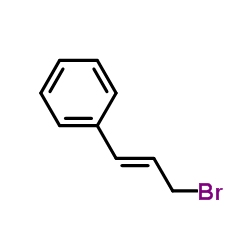 |
Cinnamyl bromide
CAS:4392-24-9 |
|
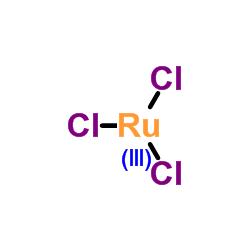 |
ruthenium chloride
CAS:10049-08-8 |