Regiodivergent Baeyer-Villiger oxidation of fused ketones by recombinant whole-cell biocatalysts.
Marko D Mihovilovic, Peter Kapitán, Petra Kapitánová
Index: ChemSusChem 1(1-2) , 143-8, (2008)
Full Text: HTML
Abstract
Recombinant Escherichia coli cells expressing monooxygenases of different bacterial origin were evaluated in microbial Baeyer-Villiger oxidations of racemic fused ketones. During the enzymatic oxidation process, both the "normal" lactone generated by migration of the more-substituted carbon atom and/or the "abnormal" lactone resulting from migration of the less-substituted carbon atom can be formed. Depending on the nature of the Baeyer-Villiger monooxygenase, either a regiodivergent biooxygenation to both lactones in high optical purities was observed or essentially racemic "normal" rearrangement product was formed. The complementary behavior of enzymes was found to correlate with our previous observation of clustering of cycloketone-oxidizing proteins into two distinct clusters based on phylogenetic relationship and biocatalyst performance.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
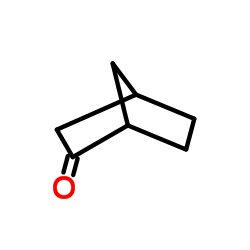 |
NORCAMPHOR
CAS:497-38-1 |
C7H10O |
|
Conformational landscape and the selectivity of cytochrome P...
2015-06-04 [J. Phys. Chem. B 119 , 6620-7, (2015)] |
|
Role of protein and substrate dynamics in catalysis by Pseud...
2002-12-10 [Biochemistry 41(49) , 14499-508, (2002)] |
|
Conformational relaxation in hemoproteins: the cytochrome P-...
2000-11-21 [Biochemistry 39(46) , 14219-31, (2000)] |
|
The effect of proton donors on the facial stereoselectivity ...
2011-03-04 [J. Org. Chem. 76(5) , 1355-60, (2011)] |
|
Calculation of electronic circular dichroism spectra with ti...
2009-01-29 [J. Phys. Chem. A 113(4) , 767-76, (2009)] |