Development and validation of a liquid chromatography-tandem mass spectrometry method for the determination of goserelin in rabbit plasma.
Min Kyung Kim, Tae Ho Lee, Joon Hyuk Suh, Han Young Eom, Jung Won Min, Hyesun Yeom, Unyong Kim, Hyung Joon Jung, Kyung Hoi Cha, Yong Seok Choi, Jeong-Rok Youm, Sang Beom Han
Index: J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 878(24) , 2235-42, (2010)
Full Text: HTML
Abstract
A rapid and sensitive liquid chromatography-electrospray ionization tandem mass spectrometry method (LC-ESI-MS/MS) was developed and validated for the determination of goserelin in rabbit plasma. Various parameters affecting plasma sample preparation, LC separation, and MS/MS detection were investigated, and optimized conditions were identified. Acidified plasma samples were applied to Oasis((R)) HLB solid-phase extraction (SPE) cartridges. Extracted samples were evaporated under a stream of nitrogen and then reconstituted with 100microL mobile phase A. The separation was achieved on a Capcell-Pak C18 (2.0mmx150mm, 5microm, AQ type) column with a gradient elution of solvent A (0.05% acetic acid in deionized water/acetonitrile=85/15; v/v) and solvent B (acetonitrile) at a flow rate of 250microL/min. The LC-MS/MS system was equipped with an electrospray ion source operating in positive ion mode. Multiple-reaction monitoring (MRM) of the precursor-product ion transitions consisted of m/z 635.7-->m/z 607.5 for goserelin and m/z 424.0-->m/z 292.1 for cephapirin (internal standard). The proposed method was validated by assessing specificity, linearity, limit of quantification (LOQ), intra- and inter-day precision and accuracy, recovery, and stability. Linear calibration curves were obtained in the concentration range of 0.1-20ng/mL (the correlation coefficients were above 0.99). The LOQ of the method was 0.1ng/mL. Results obtained from the validation study of goserelin showed good accuracy and precision at concentrations of 0.1, 1, 5, 10, and 20ng/mL. The validated method was successfully applied to a pharmacokinetic study of goserelin after a single subcutaneous injection of 3.6mg of goserelin in healthy white rabbits.Copyright 2010 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
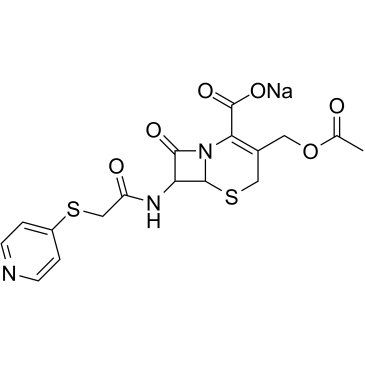 |
Cefapirin sodium
CAS:24356-60-3 |
C17H16N3NaO6S2 |
|
Validation study of the BetaStar plus lateral flow assay for...
2012-01-01 [J. AOAC Int. 95(4) , 1211-21, (2012)] |
|
Bacteriological and cytological findings during the late pue...
2010-01-01 [Acta Vet. Scand. 52 , 41, (2010)] |
|
Sorption mechanisms of cephapirin, a veterinary antibiotic, ...
2009-06-01 [Environ. Pollut. 157(6) , 1849-56, (2009)] |
|
Factors associated with the risk of antibiotic residues and ...
2009-02-16 [Vet. Microbiol. 134(1-2) , 150-6, (2009)] |
|
Short communication: Rapid antibiotic screening tests detect...
2010-09-01 [J. Dairy Sci. 93(9) , 3961-4, (2010)] |