Acta physiologica latino americana
1980-01-01
The kininases of Bothrops jararaca plasma.
A A Lavras, M Fichman, E Hiraichi, T Tobo, M A Boucault
Index: Acta Physiol. Lat. Am. 30(4) , 269-74, (1980)
Full Text: HTML
Abstract
Evidence is presented to suggest that kininase activity of Bothrops jararaca plasma is due to the presence of at least three distinct enzymes: a carboxypeptidase B type enzyme, similar to that found in human plasma in that its activity is enhanced by Co2+ (1 X 10(-4) M); a carboxypeptidase B type enzyme whose activity is unaffected by Co2+, and an enzyme which cleaves bradykinin to liberate Phe-Arg as the major peptide fragment formed. The latter enzyme is responsible for the major kininase activity of this snake plasma and is identified as a dipeptide hydrolase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
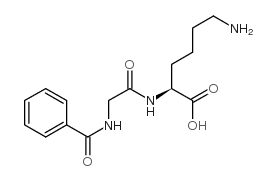 |
Hippuryl-Lys-OH
CAS:740-63-6 |
C15H21N3O4 |
Related Articles:
More...
|
Assay of carboxypeptidase N activity in serum by liquid-chro...
1985-12-01 [Clin. Chem. 31(12) , 1936-9, (1985)] |
|
Isolation and purification of creatine kinase conversion fac...
1987-02-01 [Clin. Biochem. 20(1) , 21-9, (1987)] |
|
Model studies on protein glycation: influence of cysteine on...
2008-04-01 [Ann. N. Y. Acad. Sci. 1126 , 248-52, (2008)] |
|
Human 'creatine kinase conversion factor' identified as a ca...
1984-07-15 [Biochem. J. 221(2) , 465-70, (1984)] |
|
Formation of peptide-bound Heyns compounds.
2008-04-09 [J. Agric. Food Chem. 56(7) , 2522-7, (2008)] |