International Journal of Peptide and Protein Reseach
1994-04-01
Synthesis of disulfide-bridged fragments of omega-conotoxins GVIA and MVIIA. Use of Npys as a protecting/activating group for cysteine in Fmoc syntheses.
R G Simmonds, D E Tupper, J R Harris
Index: Int. J. Pept. Protein Res. 43(4) , 363-6, (1994)
Full Text: HTML
Abstract
The 3-nitro-2-pyridinesulphenyl (Npys) moiety is finding increasing utility as a protecting-activating group for cysteine, particularly in the synthesis of cyclic and unsymmetrical disulfides using the Boc strategy. This chemistry has been extended to peptides assembled by the Fmoc strategy. N-Terminal Cys(Npys) is introduced via Boc-Cys(Npys)-OPfp. Non-N-terminal Cys(Npys) is incorporated by reacting a resin-bound, fully protected Cys(Acm) peptide with NpysCl. This approach has been applied to the synthesis of four disulfide-bridged fragments of omega-conotoxins GVIA and MVIIA.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
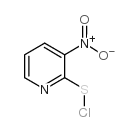 |
3-nitro-2-pyridinesulfenyl chloride
CAS:68206-45-1 |
C5H3ClN2O2S |
Related Articles:
More...
|
Synthesis and stability of 3-nitro-2-pyridinesulfenyl chlori...
1993-11-01 [Int. J. Pept. Protein Res. 42(2) , 159-64, (1993)] |
|
Discriminative affinity labelling of opioid receptors by enk...
[J. Chromatogr. A. 597(1-2) , 425-8, (1992)] |
|
Compatibility of the S-(3-nitro-2-pyridinesulfenyl) protecti...
1992-01-01 [Pept. Res. 5(5) , 262-4, (1992)] |
|
Thiolysis of the 3-nitro-2-pyridinesulfenyl (Npys) protectin...
1990-06-01 [Int. J. Pept. Protein Res. 35(6) , 545-9, (1990)] |
|
Design and synthesis of a kininogen-based selective inhibito...
1994-01-01 [Pept. Res. 7 , 32-35, (1994)] |