| Structure | Name/CAS No. | Articles |
|---|---|---|
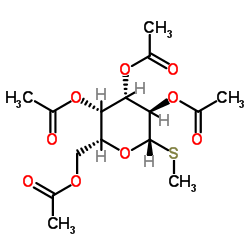 |
methyl 2,3,4,6-tetra-o-acetyl-beta-d-thiogalactopyranoside
CAS:55722-48-0 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
methyl 2,3,4,6-tetra-o-acetyl-beta-d-thiogalactopyranoside
CAS:55722-48-0 |