Organic Letters
2003-07-24
Efficient, stereoselective synthesis of oxazolo[3,2-a]pyrazin-5-ones: novel bicyclic lactam scaffolds from the bicyclocondensation of 3-aza-1,5-ketoacids and amino alcohols.
Josef R Bencsik, Timothy Kercher, Michael O'Sullivan, John A Josey
Index: Org. Lett. 5(15) , 2727-30, (2003)
Full Text: HTML
Abstract
[reaction: see text] The bicyclocondensation of 3-aza-1,5-ketoacids and amino alcohols furnished novel oxazolo[3,2-a]pyrazin-5-one scaffolds possessing angular, ring junction substituents in high yield with excellent levels of substrate-based diastereocontrol. Mild oxidation of serinol-derived scaffolds provided access to a new class of constrained dipeptide surrogates. Deprotection of the endocyclic amine contained within these scaffolds allows for further diversification via N-functionalization.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
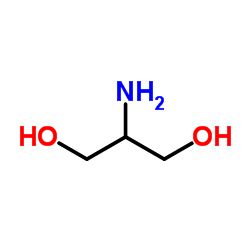 |
Serinol
CAS:534-03-2 |
C3H9NO2 |
Related Articles:
More...
|
Novel Redox-Responsive Amphiphilic Copolymer Micelles for Dr...
2015-11-01 [AAPS J. 17 , 1357-68, (2015)] |
|
Design, synthesis and characterization of novel binary V(V)-...
2015-06-01 [J. Inorg. Biochem. 147 , 99-115, (2015)] |
|
Phospholipase D-catalyzed synthesis of new phospholipids wit...
2008-04-01 [Chem. Phys. Lipids 152(2) , 71-7, (2008)] |
|
Reactions of serine palmitoyltransferase with serine and mol...
2004-02-03 [Biochemistry 43(4) , 1082-92, (2004)] |
|
Structures of a unique O-polysaccharide of Edwardsiella tard...
2012-07-01 [Carbohydr. Res. 355 , 56-62, (2012)] |