Nitrosation with Sodium Hexanitrocobaltate(III).
Bogdan Stefane, Marijan Kocevar, Slovenko Polanc
Index: J. Org. Chem. 62 , 7165, (1997)
Full Text: HTML
Abstract
Na(3)Co(NO(2))(6) has been investigated as a new reagent for the nitrosation of various substrates containing an amino functionality. Reactions took place in an aqueous solution of the reagent. The pH of the reaction mixture remained in the range 4.3-5. Thus, hydrazides were transformed to the corresponding acyl azides, and the reactions with arenesulfonyl hydrazines afforded arenesulfonyl azides. Treatment of aromatic amines with Na(3)Co(NO(2))(6) gave 1,3-diaryltriazenes in excellent yields; coupling of the initially formed diazo compound to the electron rich aromatic ring was also observed. Nitrosation of aliphatic amines was not possible due to complex formation with the reagent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
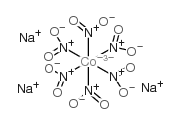 |
sodium cobaltinitrite
CAS:13600-98-1 |
CoN6Na3O12 |
|
Pseudohalide anions reveal a novel extracellular site for po...
2012-11-01 [Br. J. Pharmacol. 167(5) , 1062-75, (2012)] |
|
Indirect atomic absorption spectrometric determination of pi...
2000-03-01 [J. Pharm. Biomed. Anal. 22(2) , 235-40, (2000)] |
|
Effect of sodium hexanitrocobaltate (III) decomposition on i...
1990-01-01 [Stain Technol. 65(1) , 15-24, (1990)] |
|
[Hygienic standard basis for potassium hexanitrocobaltate in...
1980-10-01 [Gig. Sanit. (10) , 72-3, (1980)] |
|
Structure and dynamics of condensed DNA probed by 1,1'-(4,4,...
2002-12-24 [Biochemistry 41(51) , 15277-87, (2002)] |