| Structure | Name/CAS No. | Articles |
|---|---|---|
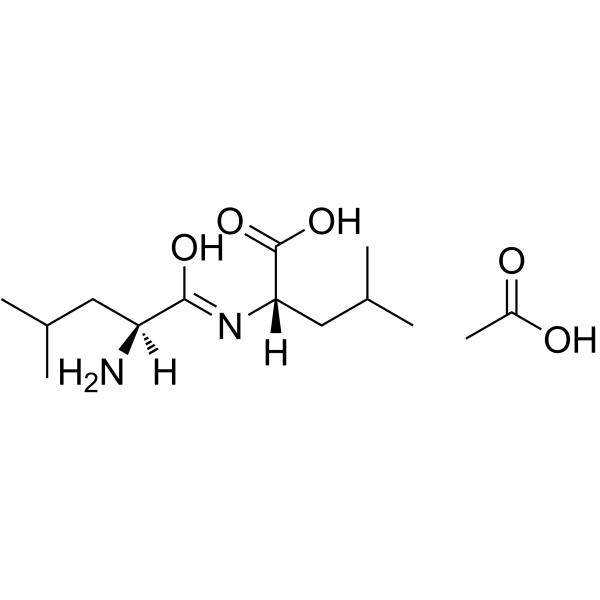 |
LEU-LEU ACETATE SALT
CAS:73237-76-0 |
|
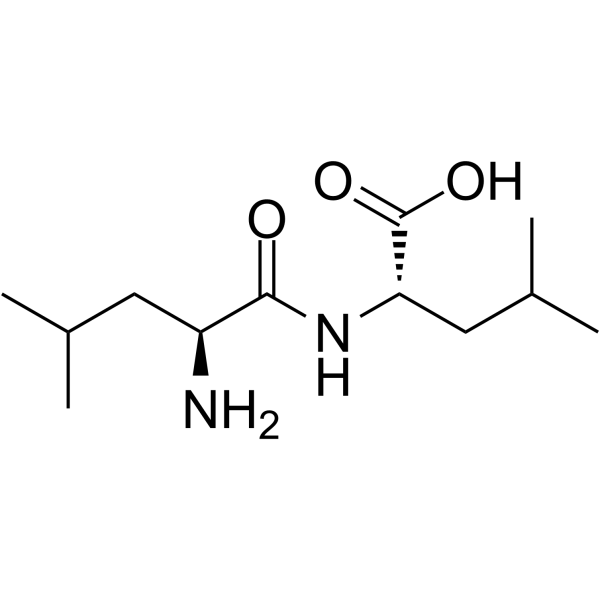 |
Leu-Leu
CAS:3303-31-9 |
|
 |
H-Leu-D-Leu-OH
CAS:17665-02-0 |