A family 5 β-mannanase from the thermophilic fungus Thielavia arenaria XZ7 with typical thermophilic enzyme features.
Haiqiang Lu, Huitu Zhang, Pengjun Shi, Huiying Luo, Yaru Wang, Peilong Yang, Bin Yao
Index: Appl. Microbiol. Biotechnol. 97(18) , 8121-8, (2013)
Full Text: HTML
Abstract
A novel β-mannanase gene, man5XZ7, was cloned from thermophilic fungus Thielavia arenaria XZ7, and successfully expressed in Pichia pastoris. The gene (1,110 bp) encodes a 369-amino acid polypeptide with a molecular mass of approximately 40.8 kDa. The deduced sequence of Man5XZ7 consists of a putative 17-residue signal peptide and a catalytic module belonging to glycoside hydrolase (GH) family 5, and displays 76 % identity with the experimentally verified GH 5 endo-β-1,4-mannanase from Podospora anserina. Recombinant Man5XZ7 was optimally active at 75 °C and pH 5.0 and exhibited high activity at a wide temperature range (>50.0 % activity at 50-85 °C). Moreover, it had good adaptability to acidic to basic pH (>74.1 % activity at pH 4.0-7.0 and 25.6 % even at pH 9.0) and good stability from pH 3.0 to 10.0. These enzymatic properties showed that Man5XZ7 was a new thermophilic and alkali-tolerant β-mannanase. Further amino acid composition analysis indicated that Man5XZ7 has several characteristic features of thermophilic enzymes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
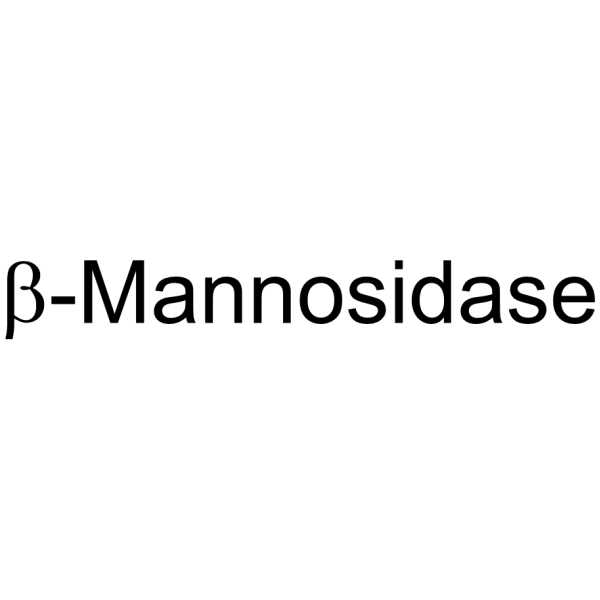 |
β-Mannosidase
CAS:9025-43-8 |
|
Expression of lignocellulolytic enzymes in Pichia pastoris.
2012-01-01 [Microb. Cell Fact. 11 , 61, (2012)] |
|
Expression and characterization of a Bifidobacterium adolesc...
2013-01-01 [Appl. Environ. Microbiol. 79(1) , 133-40, (2013)] |
|
Lipidemic responses of male broiler chickens to enzyme-suppl...
2013-11-01 [Pak. J. Biol. Sci. 16(21) , 1295-302, (2013)] |
|
Cloning, expression, and characterization of β-mannanase fro...
2013-04-01 [Appl. Biochem. Biotechnol. 169(8) , 2326-40, (2013)] |
|
Novel low-temperature-active, salt-tolerant and proteases-re...
2012-05-01 [J. Biosci. Bioeng. 113(5) , 568-74, (2012)] |