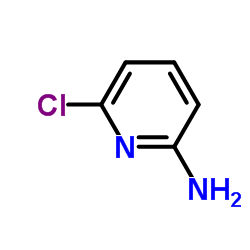
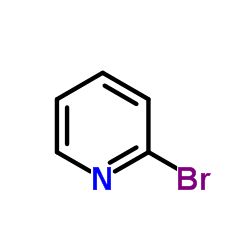
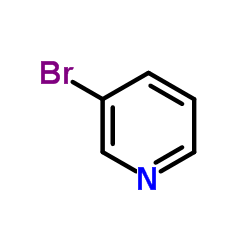
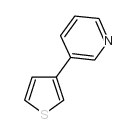
|
~9% |
|
~92% |
|
~93% |
|
~92% |
|
~83% |
|
~97% |
|
~86% |
|
~97% |
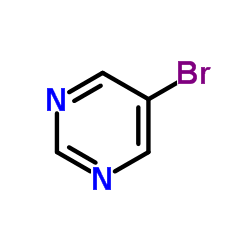
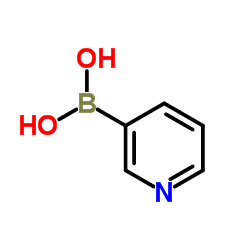
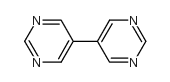

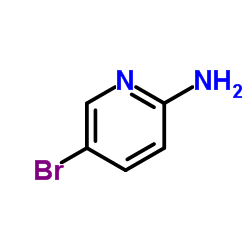
![3,3'-BIPYRIDIN]-6-AMINE Structure](https://image.chemsrc.com/caspic/167/31970-30-6.png)
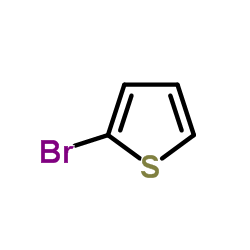

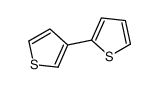
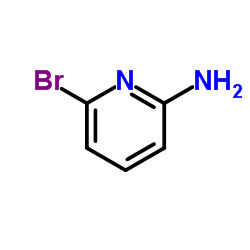
![[2,3''-Bipyridin]-6-amine Structure](https://image.chemsrc.com/caspic/459/39883-47-1.png)