| Structure | Name/CAS No. | Articles |
|---|---|---|
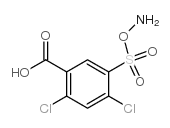 |
Protein Disulfide Isomerase, from bovine liver
CAS:37318-49-3 |
|
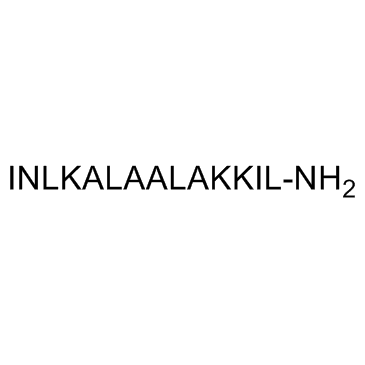 |
Mastoparan trifluoroacetate salt
CAS:72093-21-1 |