|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

![1-[AMINO-(3-NITRO-PHENYL)-METHYL]-NAPHTHALEN-2-OL Structure](https://image.chemsrc.com/caspic/250/561052-52-6.png)
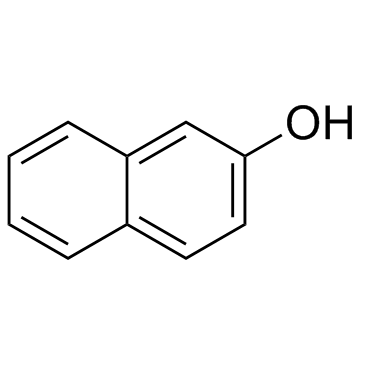
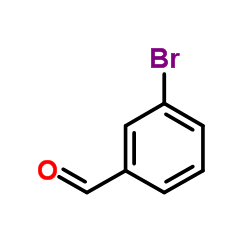
![1-[amino-(3-bromophenyl)methyl]naphthalen-2-ol Structure](https://image.chemsrc.com/caspic/174/561052-54-8.png)
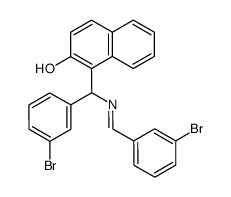
![1-[amino-(4-methoxyphenyl)methyl]naphthalen-2-ol Structure](https://image.chemsrc.com/caspic/098/6271-13-2.png)
![1-[4-methoxy-α-(4-methoxy-benzylidenamino)-benzyl]-[2]naphthol Structure](https://image.chemsrc.com/caspic/325/561053-05-2.png)
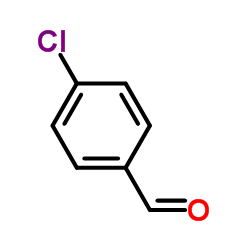
![1-[amino-(4-chlorophenyl)methyl]naphthalen-2-ol Structure](https://image.chemsrc.com/caspic/088/561052-56-0.png)