Structure-activity relationship of imidazo[1,2-a]pyridines as ligands for detecting beta-amyloid plaques in the brain.
Zhi-Ping Zhuang, Mei-Ping Kung, Alan Wilson, Chi-Wan Lee, Karl Plössl, Catherine Hou, David M Holtzman, Hank F Kung
Index: J. Med. Chem. 46(2) , 237-43, (2003)
Full Text: HTML
Abstract
A series of novel beta-amyloid (A beta) aggregate-specific ligands, 2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyridine, 16(IMPY), and its related derivatives were prepared. An in vitro binding study with preformed A beta aggregates showed that 16(IMPY) and its bromo derivative competed with binding of 2-(4'-dimethylaminophenyl)-6-iodobenzothiazole, [125I]7(TZDM), a known ligand for A beta aggregates, with high binding affinities (K(i) = 15 and 10 nM, respectively). In vitro autoradiography of brain sections of a transgenic mouse (Tg2576) with [125I]16(IMPY) displayed high selective binding to amyloid-like structures, comparable to that observed by staining with thioflavin-S visualized under fluorescence. In vivo biodistribution after an intravenous injection of [125I]16(IMPY) in normal mice showed a high initial brain uptake and fast washout, indicating a low background activity associated with this iodinated ligand. Taken together, the data suggests that [123I]16(IMPY) may be useful for imaging A beta aggregates in patients with Alzheimer's disease.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
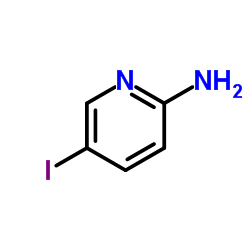 |
5-Iodo-2-pyridinamine
CAS:20511-12-0 |
C5H5IN2 |
|
Methyl 6-(phenylsulfinyl)imidazo[1,2-a]pyridine-2-carbamate,...
1978-02-01 [J. Med. Chem. 21(2) , 235-7, (1978)] |
|
Vibrational spectra and assignments of 2-amino-5-iodopyridin...
2007-07-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 67(3-4) , 830-6, (2007)] |
|
Substituted 2-Sulfonamido-5-aminopyridines. II. Caldwell WT,...
[J. Am. Chem. Soc. 66(9) , 1479-1484, (1944)] |
|
Transition metal halide salts of 2-amino-5-substituted-pyrid...
[J. Coord. Chem. 55(7) , 795-803, (2002)] |