Substituted N-(3,5-dichlorobenzenesulfonyl)-L-prolyl-phenylalanine analogues as potent VLA-4 antagonists.
Ihor E Kopka, David N Young, Linus S Lin, Richard A Mumford, Plato A Magriotis, Malcolm MacCoss, Sander G Mills, Gail Van Riper, Ermengilda McCauley, Linda E Egger, Usha Kidambi, John A Schmidt, Kathryn Lyons, Ralph Stearns, Stella Vincent, Adria Colletti, Zhen Wang, Sharon Tong, Junying Wang, Song Zheng, Karen Owens, Dorothy Levorse, William K Hagmann
Index: Bioorg. Med. Chem. Lett. 12(4) , 637-40, (2002)
Full Text: HTML
Abstract
A series of substituted N-(3,5-dichlorobenzenesulfonyl)-L-prolyl- and alpha-methyl-L-prolyl-phenylalanine derivatives was prepared as VLA-4/VCAM antagonists. The compounds showed excellent potency with a wide variety of neutral, polar, electron withdrawing or donating groups on the phenylalanine ring (IC50 approximately 1 nM). Heteroaryl ring substitution for phenylalanine was also well tolerated. Pharmacokinetic studies in rat were performed on a representative set of compounds in both series.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
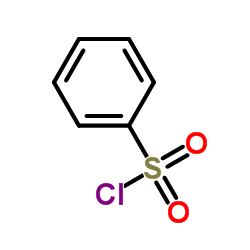 |
chlorophenylsulfone
CAS:98-09-9 |
C6H5ClO2S |
|
Design, synthesis and structure-activity relationship studie...
2014-12-01 [J. Enzyme Inhib. Med. Chem. 29(6) , 846-67, (2014)] |
|
Design, synthesis, and bioevaluation of paeonol derivatives ...
2015-01-27 [Eur. J. Med. Chem. 90 , 428-35, (2015)] |
|
Comparison of manual and benzenesulfonyl chloride-semiautoma...
1981-05-01 [J. Assoc. Off. Anal. Chem. 64(3) , 616-22, (1981)] |
|
2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alter...
2005-05-06 [Angew. Chem. Int. Ed. Engl. 44(19) , 2922-5, (2005)] |
|
Microsphere formation in a series of derivatized alpha-amino...
1995-10-13 [J. Med. Chem. 38(21) , 4257-62, (1995)] |