Bioscience, Biotechnology, and Biochemistry
2002-02-01
Kinetic resolutions of indan derivatives using bacteria.
Naoki Tarui, Hayao Watanabe, Kohji Fukatsu, Shigenori Ohkawa, Kazuo Nakahama
Index: Biosci. Biotechnol. Biochem. 66(2) , 464-6, (2002)
Full Text: HTML
Abstract
Racemic indan derivatives have been resolved by the hydrolysis of amide bonds using Corynebacterium ammoniagenes IFO12612 to produce (S)-amine and (R)-amides. In the kinetic resolution of 1 (N-12-(6-methoxy-indan-1-yl)ethyl]acetamide), it was possible to run the reaction to 44% conversion on a 10-g scale, obtaining (S)-amine 4 ((S)-2-(6-methoxy-indan-1-yl)ethylamine) at >99% enantiomeric excess (ee) and (R)-1 at 98% ee.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
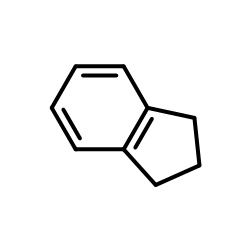 |
Indane
CAS:496-11-7 |
C9H10 |
Related Articles:
More...
|
Bicyclic naphthenic acids in oil sands process water: identi...
2015-01-23 [J. Chromatogr. A. 1378 , 74-87, (2015)] |
|
Desaturation, dioxygenation, and monooxygenation reactions c...
1995-05-01 [J. Bacteriol. 177(10) , 2615-21, (1995)] |
|
Predictive three-dimensional quantitative structure-activity...
2005-06-02 [J. Med. Chem. 48 , 3808-15, (2005)] |
|
Aromatic hydroxylation of indan by o-xylene-degrading Rhodoc...
2010-01-01 [Appl. Environ. Microbiol. 76(1) , 375-7, (2010)] |
|
Selective inhibition of carboxylesterases by isatins, indole...
2007-04-19 [J. Med. Chem. 50 , 1876-85, (2007)] |