| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
4-Bromo-L-Phe-OH
CAS:14091-15-7 |
|
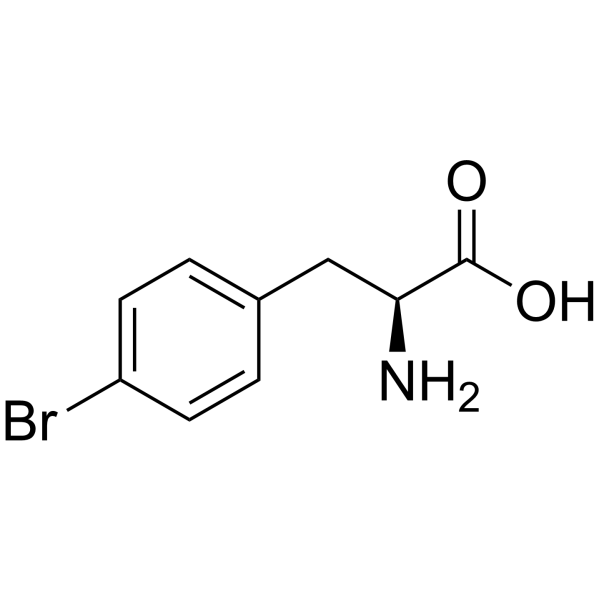 |
H-Phe(4-Br)-OH
CAS:24250-84-8 |