Intracellular free zinc and zinc buffering in human red blood cells.
T J Simons
Index: J. Membr. Biol. 123(1) , 63-71, (1991)
Full Text: HTML
Abstract
Zn2+ has been allowed to equilibrate across the red cell membrane using two agents that increase membrane permeability to this ion: the ionophore A23187 and the specific carrier ethylmaltol. Extracellular free Zn2+ was controlled with EGTA (1,2-di(2-aminoethoxy)ethane-NNN'N'tetra-acetic acid] buffers, except in the case of ethylmaltol, which itself acts as a buffer. Measurement of cellular zinc content at different levels of free Zn2+ facilitated the study of intracellular Zn2+ binding. It was also possible to estimate intracellular free Zn2+ concentration in untreated cells using a "null-point" technique. Intracellular zinc was found to consist of an inexchangeable component of about 129 mumol/10(13) cells and an exchangeable component of 6.7 +/- 1.5 mumol/10(13) cells, with a free concentration of about 2.4 x 10(-11) M. The main component of Zn2+ buffering is hemoglobin, with a dissociation constant of about 2 x 10(-8) M.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
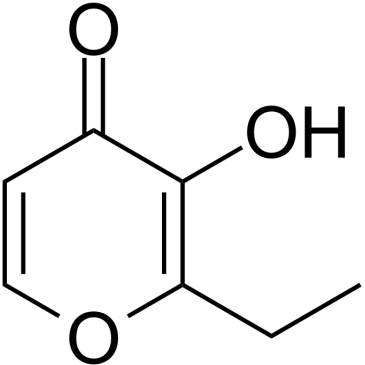 |
Ethyl maltol
CAS:4940-11-8 |
C7H8O3 |
|
Perceptual blending in odor mixtures depends on the nature o...
2012-02-01 [Chem. Senses 37(2) , 159-66, (2012)] |
|
Vanadium treatment of type 2 diabetes: A view to the future
2009-01-01 [J. Inorg. Biochem. 103(4) , 554-8, (2009)] |
|
Reversed-phase high-performance liquid chromatography of non...
1994-08-05 [J. Chromatogr. B, Biomed. Appl. 658(1) , 121-7, (1994)] |
|
Facilitated uptake of zinc into human erythrocytes. Relevanc...
1990-03-15 [Biochem. Pharmacol. 39(6) , 1005-12, (1990)] |
|
Characteristics of iron(III) uptake by isolated fragments of...
1988-05-15 [Biochem. Pharmacol. 37(10) , 2051-7, (1988)] |