Kinetic mechanism of ketoreductase activity of prostaglandin F synthase from bovine lung.
O A Barski, K Watanabe
Index: FEBS Lett. 320(2) , 107-10, (1993)
Full Text: HTML
Abstract
The kinetic mechanism of ketoreductase activity of bovine lung prostaglandin F synthase, expressed in E. coli, was investigated. Data on initial velocity and radioisotope exchange between [3H]prostaglandin D2 and 9 alpha,11 beta-prostaglandin F2 suggest that the enzyme obeys the ping-pong mechanism. Using a fluorescence technique we obtained a binding constant of 3 microM for NADPH. This is in close correlation with the kinetically determined intrinsic Michaelis constant for NADPH. Activation energy of the redox process was determined from the temperature dependence of maximal velocities for nitrobenzaldehyde and menadione and was found to be 119 and 96 kJ/mol, respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
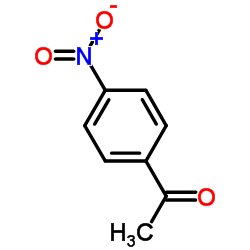 |
4-Nitroacetophenone
CAS:100-19-6 |
C8H7NO3 |
|
Analysis of the Photodegradation of the Imidazolinone Herbic...
2015-12-23 [J. Agric. Food Chem. 63 , 10768-77, (2015)] |
|
[Semiconservative and unscheduled DNA-synthesis of rat thymo...
1982-02-01 [Strahlentherapie 158(2) , 112-22, (1982)] |
|
Structure-reactivity effects on primary deuterium isotope ef...
2009-10-07 [J. Am. Chem. Soc. 131(39) , 13952-62, (2009)] |
|
Modification of radiation sensitivity of Bacillus megaterium...
1974-06-01 [Radiat. Res. 58(3) , 481-8, (1974)] |
|
Metabolic potentiation of the radiosensitization of hypoxic ...
1983-03-01 [Radiat. Res. 93(3) , 516-24, (1983)] |