| Structure | Name/CAS No. | Articles |
|---|---|---|
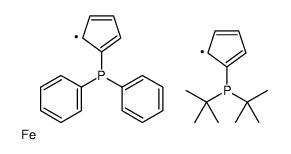 |
1-diphenylphosphino-1'-(di-tert-butylph&
CAS:95408-38-1 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
1-diphenylphosphino-1'-(di-tert-butylph&
CAS:95408-38-1 |