Biological activity of modified and exchanged 2-amino-5-nitrothiazole amide analogues of nitazoxanide.
T Eric Ballard, Xia Wang, Igor Olekhnovich, Taylor Koerner, Craig Seymour, Paul S Hoffman, Timothy L Macdonald
Index: Bioorg. Med. Chem. Lett. 20(12) , 3537-9, (2010)
Full Text: HTML
Abstract
Head group analogues of the antibacterial and antiparasitic drug nitazoxanide (NTZ) are presented. A library of 39 analogues was synthesized and assayed for their ability to suppress growth of Helicobacter pylori, Campylobacter jejuni, Clostridium difficile and inhibit NTZ target pyruvate:ferredoxin oxidoreductase (PFOR). Two head groups assayed recapitulated NTZ activity and possessed improved activity over their 2-amino-5-nitrothiazole counterparts, demonstrating that head group modification is a viable route for the synthesis of NTZ-related antibacterial analogues.Copyright 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
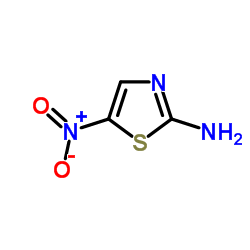 |
5-Nitrothiazol-2-amine
CAS:121-66-4 |
C3H3N3O2S |
|
Evaluation of 2-amino-5-nitrothiazole as a hypoxic cell radi...
1982-06-01 [Radiat. Res. 90(3) , 575-85, (1982)] |
|
Platinum complexes with one radiosensitizing ligand [PtCl2(N...
1987-11-01 [Radiat. Res. 112(2) , 273-82, (1987)] |
|
2-amino-5-nitrothiazole.
1983-07-01 [IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 31 , 71-7, (1983)] |
|
Reaction of 2-amino-5-nitrothiazole with palladium salts. Fa...
[Inorganica Chim. Acta 126(2) , 137-139, (1987)] |
|
Analysis of Oligonucleotide and Protein via Matrix-assisted ...
[Chem. Res. Chin. Univ. 12 , 012, (2004)] |