Direct asymmetric hydrogenation of 2-oxo-4-arylbut-3-enoic acids.
Lvfeng Zhu, Qinghua Meng, Weizheng Fan, Xiaomin Xie, Zhaoguo Zhang
Index: J. Org. Chem. 75(17) , 6027-30, (2010)
Full Text: HTML
Abstract
A challenging direct asymmetric hydrogenation of (E)-2-oxo-4-arylbut-3-enoic acids to give 2-hydroxy-4-arylbutanoic acids (85.4-91.8% ee) was achieved with a Ru catalyst based on SunPhos as the chiral ligand. Further investigation of the reaction revealed that partial isomerization of 2-hydroxy-4-arylbutenoic acids was involved in the hydrogenation process. Employing the reaction conditions to the hydrogenation of 2-oxo-4-phenylbutanoic acid resulted in better enantioselectivity (91.8% ee) and efficiency (TON = 2000, TOF = 200 h(-1)), which offers a useful method for the synthesis of a common intermediate for ACE inhibitors.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
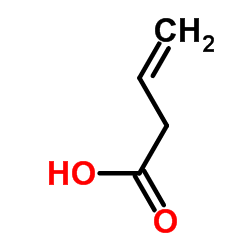 |
3-Butenoic acid
CAS:625-38-7 |
C4H6O2 |
|
On the Question of Site-Selective Ligand Exchange in Carboxy...
2015-08-01 [Eur. J. Inorg. Chem. 2015 , 2889-2894, (2015)] |
|
Synthesis of base substituted 2-hydroxy-3-(purin-9-yl)-propa...
2003-12-01 [Nucleosides Nucleotides Nucleic Acids 22(12) , 2145-60, (2003)] |
|
Increases in microbial nitrogen production and efficiency in...
2007-04-01 [Can. J. Microbiol. 53(4) , 496-503, (2007)] |
|
Domino metathesis of 3,6-dihydro-1,2-oxazine: access to isox...
2007-04-12 [Org. Lett. 9 , 1485, (2007)] |
|
4-Phenyl-3-butenoic acid, an in vivo inhibitor of peptidylgl...
1990-04-30 [Eur. J. Biochem. 189(2) , 363-8, (1990)] |