Synthesis and in vitro antiproliferative activity of new phenylaminoisoquinolinequinones against cancer cell lines.
Virginia Delgado, Andrea Ibacache, Verónica Arancibia, Cristina Theoduloz, Jaime A Valderrama
Index: Molecules 18(1) , 721-34, (2013)
Full Text: HTML
Abstract
A variety of phenylaminoisoquinolinequinones were synthesized and tested for their antiproliferative activity against three human-tumor derived cancer cell lines. The new aminoquinones were prepared from 4-methoxycarbonyl-3-methylisoquinoline-5,8-quinone (1) via acid-induced amination and bromination reactions. Remarkable differences in antiproliferative activity were observed depending upon the location and donor capacity of the substituted phenylamino group at the quinone nucleus. The effect of the substituents on the biological activity is discussed in terms of the donor-acceptor interactions which were evaluated through the redox properties of the aminoquinones.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
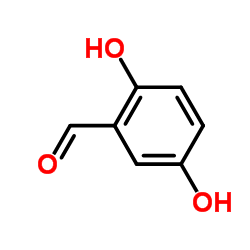 |
2,5-Dihydroxybenzaldehyde
CAS:1194-98-5 |
C7H6O3 |
|
Liquid chromatographic/electrospray ionization mass spectrom...
2010-03-15 [Rapid Commun. Mass Spectrom. 24(5) , 634-42, (2010)] |
|
A bioanode based on MWCNT/protein-assisted co-immobilization...
2010-07-15 [Biosens. Bioelectron. 25(11) , 2515-21, (2010)] |
|
The reductive half-reaction of xanthine oxidase. Reaction wi...
1999-02-05 [J. Biol. Chem. 274(6) , 3323-30, (1999)] |
|
Sequential C-N and C-O bond formation in a single pot: synth...
2012-01-20 [Org. Lett. 14(2) , 552-5, (2012)] |
|
Analysis of aliphatic amines in air samples by HPLC with ele...
2002-08-01 [J. Pharm. Biomed. Anal. 29(6) , 1105-11, (2002)] |