Synthesis of apiose-containing oligosaccharide fragments of the plant cell wall: fragments of rhamnogalacturonan-II side chains A and B, and apiogalacturonan.
Sergey A Nepogodiev, Margherita Fais, David L Hughes, Robert A Field
Index: Org. Biomol. Chem. 9(19) , 6670-84, (2011)
Full Text: HTML
Abstract
Fragments of pectic polysaccharides rhamnogalacturonan-II (RG-II) and apiogalacturonan were synthesised using p-tolylthio apiofuranoside derivatives as key building blocks. Apiofuranose thioglycosides can be conveniently prepared by cyclization of the corresponding dithioacetals possessing a 2,3-O-isopropylidene group, which is required for preservation of the correct (3R) configuration of the apiofuranose ring. The remarkable stability of this protecting group in apiofuranose derivatives requires its replacement with a more reactive protecting group, such as a benzylidene acetal which was used in the synthesis of trisaccharide β-Rhap-(1→3')-β-Apif-(1→2)-α-GalAp-OMe. The X-ray crystal structure of the protected precursor of this trisaccharide has been elucidated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
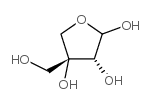 |
D-apiose
CAS:639-97-4 |
C5H10O5 |
|
Use of Commercial Dry Yeast Products Rich in Mannoproteins f...
2015-06-17 [J. Agric. Food Chem. 63 , 5670-81, (2015)] |
|
The acetylation of apiitol in the determination of apiose.
1990-05-15 [Carbohydr. Res. 199(1) , 55-65, (1990)] |
|
cDNA-AFLP analysis on bolting or flowering of flowering Chin...
2012-07-01 [Mol. Biol. Rep. 39(7) , 7525-31, (2012)] |
|
Synthesis and in vitro activity of 4' and 5'-modified analog...
2009-11-01 [Nucleosides Nucleotides Nucleic Acids 28(11) , 1104-16, (2009)] |
|
Simple synthesis and anti-HIV activity of novel 3'-vinyl bra...
2006-01-01 [Nucleosides Nucleotides Nucleic Acids 25(8) , 871-8, (2006)] |