| Structure | Name/CAS No. | Articles |
|---|---|---|
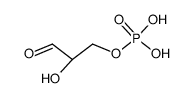 |
DL-glyceraldehyde 3-phosphate
CAS:20283-52-7 |
A Hall, J R Knowles
Index: Biochemistry 14(19) , 4348-53, (1975)
Full Text: HTML
By a combination of methods involving enzyme-catalyzed reactions and classical iodination techniques it has been possible to obtain all the relevant rate constants for the uncatalyzed interconversion of dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate via their common enediol intermediate. These rate constants are compared with those for the individual steps of the triosephosphate isomerase catalyzed reaction, and a quantitative picture of the effectiveness of the enzyme as a catalyst has been delineated. It is apparent that the enzyme increases the enolization rate of dihydroxyacetone phosphate by a factor of more than 10(9) over that of the uncatalyzed reaction.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
DL-glyceraldehyde 3-phosphate
CAS:20283-52-7 |
C3H7O6P |
|
L-Glyceraldehude 3-phosphate, a bactericidal agent.
1977-01-01 [Antimicrob. Agents Chemother. 11(1) , 147-53, (1977)] |
|
Reaction of triosephosphate isomerase with L-glyceraldehyde ...
1985-02-12 [Biochemistry 24(4) , 949-53, (1985)] |
|
L-glyceraldehyde 3-phosphate reductase from Escherichia coli...
2010-02-01 [Bioorg. Chem. 38(1) , 37-41, (2010)] |
|
Purification and properties of NADP-dependent non-phosphoryl...
[Biochim. Biophys. Acta 925 , 1-10, (1987)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved