Oxidation of 3,4-dihydroxybenzylamine affords 3,4-dihydroxybenzaldehyde via the quinone methide intermediate.
M Sugumaran
Index: Pigment Cell Res. 8(5) , 250-4, (1995)
Full Text: HTML
Abstract
Dopamine and related compounds are known to be toxic to melanoma cells. Some of their toxicity may be related, in part, to the oxidation products generated from them upon their interaction with melanogenic enzymes. In this paper, we present our studies on the oxidation chemistry of 3,4-dihydroxybenzylamine, the lower homolog of dopamine. Mushroom tyrosinase catalyzed oxidation of 3,4-dihydroxybenzylamine rapidly generated the corresponding quinone. However, aminomethyl-o-benzoquinone thus formed did not accumulate in the reaction mixture, but readily transformed to another product that exhibited absorbance maxima at 280 and 310 nm. This compound was identified to be 3,4-dihydroxybenzaldehyde based on its HPLC elution profile, cochromatography with authentic sample and UV spectral properties. Possible mechanism for the formation 3,4-dihydroxybenzaldehyde from 3,4-dihydroxybenzylamine and the nature of cytotoxic quninonoid intermediates formed are discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
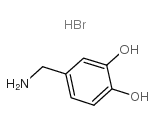 |
3,4-DIHYDROXYBENZYLAMINE HYDROBROMIDE
CAS:16290-26-9 |
C7H10BrNO2 |
|
Simultaneous determination of electroactive and non-electroa...
2010-09-23 [Anal. Chim. Acta 678(1) , 39-43, (2010)] |
|
Selective extraction of catecholamines by packed fiber solid...
2015-01-01 [Biomed. Chromatogr. 29(1) , 103-9, (2014)] |
|
ERK5 induces ankrd1 for catecholamine biosynthesis and homeo...
2016-03-01 [Cell. Signal. 28 , 177-89, (2016)] |
|
Application of a novel, plastic formed carbon as a precolumn...
1995-12-22 [J. Chromatogr. A. 718(2) , 267-72, (1995)] |
|
A method for the measurement of S-adenosylmethionine in smal...
1989-09-01 [Anal. Biochem. 181(2) , 331-5, (1989)] |