[Toxicologic evaluation of propyneb on the Wistar rat].
R Vachkova-Petrova, L Vassileva, G Antov, N Choumkov, N Dontchev, M Stavreva, E Tyagounenko, S Dinoeva, J Halkova, L Ivanova-Tchemichanska
Index: J. Toxicol. Clin. Exp. 11(7-8) , 407-16, (1991)
Full Text: HTML
Abstract
This experiment was performed in Wistar rats of both sexes exposed subchronically to 1:100, 1:500, 1:1000 and 1:1500 LD50. The evaluation was based on endpoints measured on the 30th and 90th after starting exposure and after a recovery period of 30 days: these included clinical signs, functional changes, hematological parameters, urine analysis, biochemical, histochemical, immunomorphological endpoints, electron microscopy of internal organs, chromosome examination of bone marrow. A high lethality was shown to occur with a characteristic clinical picture: interruption of weight gain, behavioural changes, leucopenia mainly involving neutrophil leucocytes, biochemical changes characteristic of liver, cardio-vascular system (myocardium and aorta) together with pathologic, biochemical, histochemical and ultrastructural changes in liver, brain, thyroid gland, myocardium, spleen and bone marrow. Endpoints were shown to be clearly dose-dependently related with small variations with the low dose, i.e. 1:1500 LD50 (5 mg/kg-1 bw).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
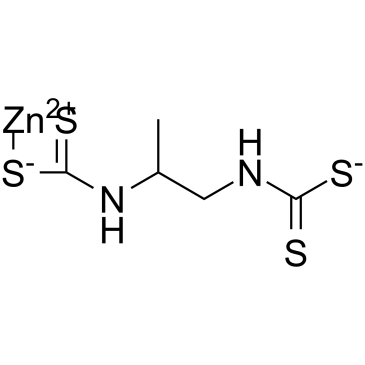 |
propineb
CAS:12071-83-9 |
C5H8N2S4Zn |
|
[Teratogenic and goitrogenic activity of propineb and propyl...
1985-02-28 [Boll. Soc. Ital. Biol. Sper. 61(2) , 271-8, (1985)] |
|
Dithiocarbamate pesticides: activity of Propineb in the micr...
1984-03-01 [Mutat. Res. 135(3) , 193-7, (1984)] |
|
Determination of dithiocarbamate fungicide propineb and its ...
2007-08-01 [Chemosphere 68(11) , 2104-10, (2007)] |
|
Effects of the dithiocarbamate fungicide propineb in primary...
2002-07-01 [Arch. Toxicol. 76(7) , 414-22, (2002)] |
|
Effects of some pesticides on the vital organs of juvenile r...
2010-12-01 [Tissue Cell 42(6) , 376-82, (2010)] |