Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans.
G Eisenhofer, A Aneman, D Hooper, B Rundqvist, P Friberg
Index: J. Neurochem. 66 , 1573, (1996)
Full Text: HTML
Abstract
This study used regional differences in plasma concentrations of norepinephrine and its metabolites to examine how production of the transmitter by sympathetic nerves, in particular, those innervating mesenteric organs, is integrated with metabolism by the liver and elimination by the kidneys. Higher concentrations of norepinephrine, its glycol metabolites 3,4-dihydroxyphenylglycol and 3-methoxy-4-hydroxyphenylglycol and their sulfate conjugates in portal venous than arterial plasma indicate substantial production of norepinephrine by mesenteric organs (15.5 nmol/min). Much lower concentrations of norepinephrine and its glycol metabolites in plasma leaving than entering the liver indicate their efficient hepatic removal (20 nmol/min). Higher concentrations of vanillylmandelic acid in the hepatic outflow than inflow indicate that this metabolic end product is produced largely from the norepinephrine and glycol metabolites removed by the liver. Renal elimination of vanillylmandelic acid (18-20 nmol/min), produced mainly by the liver (17 nmol/min), and of 3-methoxy-4-hydroxyphenylglycol sulfate (7-9 nmol/min), produced largely by mesenteric organs (7 nmol/min), compromised 86-91% of the total renal elimination of norepinephrine metabolites. The results show that mesenteric organs produce about one-half of the norepinephrine formed in the body. The liver removes substantial amounts of circulating norepinephrine and its glycol metabolites and converts these compounds to vanillylmandelic acid, which is then eliminated from the body by the kidneys. The sulfate conjugates are also metabolic end products eliminated by the kidneys. However, these metabolites are produced by extrahepatic tissues, in particular, mesenteric organs, which represent a significant source of sulfate-conjugated norepinephrine and 3,4-dihydroxyphenylglycol, and the main source of sulfate-conjugated 3-methoxy-4-hydroxyphenylglycol.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
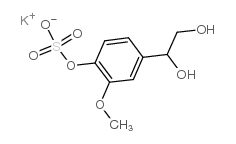 |
4-Hydroxy-3-methoxyphenylglycol sulfate potassium salt
CAS:71324-20-4 |
C9H11KO7S |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
Fluoxetine increases norepinephrine release in rat hypothala...
1997-01-01 [J. Neural Transm. Gen. Sect. 104 , 953-966, (1997)] |
|
The effect of acute, chronic and chronic intermittent stress...
1997-01-01 [Pharmacol. Biochem. Behav. 57 , 207-214, (1997)] |