| Structure | Name/CAS No. | Articles |
|---|---|---|
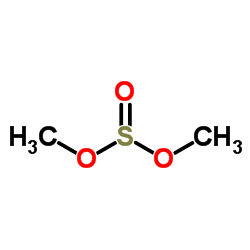 |
Dimethyl sulfite
CAS:616-42-2 |
M S Brody, R Hille
Index: Biochim. Biophys. Acta 1253(2) , 133-5, (1995)
Full Text: HTML
We have undertaken a steady-state and rapid kinetic study of the reaction of enzyme with sulfite and dimethylsulfite. Methylation of sulfite results in a significant increase in Km and Kd for the substrate in the course of steady-state and rapid reaction kinetics, respectively, but kcat and the limiting rate constant for enzyme reduction (kred) are essentially unchanged. This indicates that while substrate oxyanion groups are effective in stabilizing the Eox.S complex, the breakdown of this complex proceeds at the same rate even in their absence. The critical element of the substrate required for reactivity is a suitable lone-pair available to undertake nucleophilic attack on a Mo = O group of the active site.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Dimethyl sulfite
CAS:616-42-2 |
C2H6O3S |
|
Matrix isolation FTIR spectroscopic and theoretical study of...
2005-04-28 [J. Phys. Chem. A 109(16) , 3578-86, (2005)] |
|
[Effect of design and operation parameters on volatile alkyl...
2010-02-01 [Huan Jing Ke Xue 31(2) , 345-51, (2010)] |
|
Oxoanionic or sulfur lone pair attack? The difference in rea...
2001-09-21 [Chem. Commun. (Camb.) (18) , 1786-7, (2001)] |
|
Conformational cooling and conformation selective aggregatio...
[J. Mol. Struct. 794(1) , 196-203, (2006)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved