Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro.
Lynn S Adams, Yanjun Zhang, Navindra P Seeram, David Heber, Shiuan Chen
Index: Cancer Prev. Res. (Phila.) 3(1) , 108-13, (2010)
Full Text: HTML
Abstract
Estrogen stimulates the proliferation of breast cancer cells and the growth of estrogen-responsive tumors. The aromatase enzyme, which converts androgen to estrogen, plays a key role in breast carcinogenesis. The pomegranate fruit, a rich source of ellagitannins (ET), has attracted recent attention due to its anticancer and antiatherosclerotic properties. On consumption, pomegranate ETs hydrolyze, releasing ellagic acid, which is then converted to 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one ("urolithin") derivatives by gut microflora. The purpose of this study was to investigate the antiaromatase activity and inhibition of testosterone-induced breast cancer cell proliferation by ET-derived compounds isolated from pomegranates. A panel of 10 ET-derived compounds including ellagic acid, gallagic acid, and urolithins A and B (and their acetylated, methylated, and sulfated analogues prepared in our laboratory) were examined for their ability to inhibit aromatase activity and testosterone-induced breast cancer cell proliferation. Using a microsomal aromatase assay, we screened the panel of ET-derived compounds and identified six with antiaromatase activity. Among these, urolithin B (UB) was shown to most effectively inhibit aromatase activity in a live cell assay. Kinetic analysis of UB showed mixed inhibition, suggesting more than one inhibitory mechanism. Proliferation assays also determined that UB significantly inhibited testosterone-induced MCF-7aro cell proliferation. The remaining test compounds also exhibited antiproliferative activity, but to a lesser degree than UB. These studies suggest that pomegranate ET-derived compounds have potential for the prevention of estrogen-responsive breast cancers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
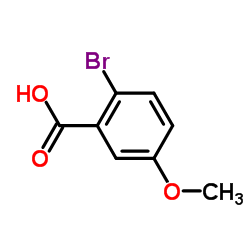 |
2-Bromo-5-methoxybenzoic acid
CAS:22921-68-2 |
C8H7BrO3 |
|
Synthesis of substituted aminobenzacridines.
1946-08-01 [J. Am. Chem. Soc. 68 , 1599-1602, (1946)] |
|
Potential metabolites of clorotepin: 2-and 3-hydroxy derivat...
[Collect. Czech. Chem. Commun. 39(12) , 3548-3559., (1974)] |
|
Design, synthesis and biological evaluation of the novel iso...
[J. Chem. Res. (M) 6(9) , 256-60, (2014)] |