Organic & Biomolecular Chemistry
2009-11-07
Total synthesis of methymycin.
Hong-Se Oh, Richeng Xuan, Han-Young Kang
Index: Org. Biomol. Chem. 7(21) , 4458-63, (2009)
Full Text: HTML
Abstract
Methynolide and 10-epi-methynolide were synthesized from the necessary segments, which were prepared by the addition of Grignard reagents to the corresponding alpha-alkoxyketones utilizing 1,2-stereochemical selection based on Cram chelation control. Ring-closing metathesis, as the key reaction, was carried out to combine the segments for the synthesis of methynolide and 10-epi-methynolide. The total synthesis of methymycin was also achieved by the glycosylation of methynolide with the trichloroimidate derivative of D-desosamine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
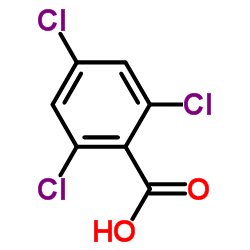 |
2,4,6-Trichlorobenzoic acid
CAS:50-43-1 |
C7H3Cl3O2 |
Related Articles:
More...
|
Influence of organic carbon and metal oxide phases on sorpti...
2015-01-01 [Environ. Monit. Assess. 187(1) , 4170, (2015)] |
|
Phenoxy herbicides and fibrates potently inhibit the human c...
2009-11-12 [J. Med. Chem. 52 , 6931-5, (2009)] |
|
Impact of nutrient composition on a degradative biofilm comm...
1997-06-01 [Appl. Environ. Microbiol. 63(6) , 2432-8, (1997)] |
|
Oral toxicity of trichlorobenzyl chloride and its acid deriv...
1987-02-01 [J. Appl. Toxicol. 7(1) , 67-70, (1987)] |
|
2, 4, 6-Trichlorobenzoic acid: Structure and hydrogen-bondin...
[Acta Crystallogr. C Struct. Chem. 52(7) , 1801-4, (1996)] |