| Structure | Name/CAS No. | Articles |
|---|---|---|
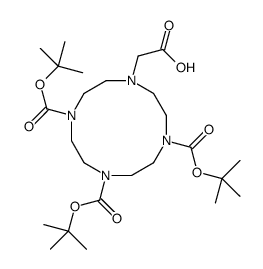 |
(4,7,10-TRI-BOC-1,4,7,10-TETRAAZACYCLODECAN-1-YL)ACETIC ACID
CAS:247193-74-4 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(4,7,10-TRI-BOC-1,4,7,10-TETRAAZACYCLODECAN-1-YL)ACETIC ACID
CAS:247193-74-4 |