Organic & Biomolecular Chemistry
2006-01-21
Enantioselective total syntheses of (-)-clasto-lactacystin beta-lactone and 7-epi-(-)-clasto-lactacystin beta-lactone.
Christopher J Hayes, Alexandra E Sherlock, Matthew D Selby
Index: Org. Biomol. Chem. 4(2) , 193-5, (2006)
Full Text: HTML
Abstract
An alkylidene carbene 1,5-CH insertion has been used as a key step in an efficient enantioselective total synthesis of (-)-clasto-lactacystin beta-lactone, and its C7-epimer. An additional noteworthy feature of the synthesis is the use of a novel oxidative deprotection procedure, utilizing DMDO, for the conversion of a late-stage benzylidene acetal into a primary alcohol and a secondary benzoate ester.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
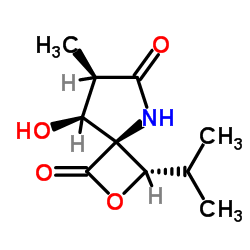 |
Omuralide
CAS:154226-60-5 |
C10H15NO4 |
Related Articles:
More...
|
Proteasome inhibitor differentially regulates expression of ...
2009-06-01 [Virus Res. 142(1-2) , 68-77, (2009)] |
|
Turnover of StAR protein: roles for the proteasome and mitoc...
2007-02-01 [Mol. Cell. Endocrinol. 265-266 , 51-8, (2007)] |
|
Targeting of a chlamydial protease impedes intracellular bac...
2011-09-01 [PLoS Pathog. 7(9) , e1002283, (2011)] |
|
Activity dependent protein degradation is critical for the f...
2011-01-01 [PLoS ONE 6(9) , e24349, (2011)] |
|
Proteasome inhibition triggers activity-dependent increase i...
2006-11-01 [J. Neurosci. 26(44) , 11333-41, (2006)] |