| Structure | Name/CAS No. | Articles |
|---|---|---|
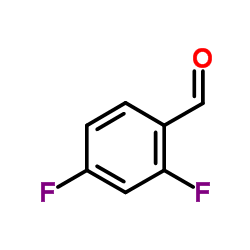 |
2,4-Difluorobenzaldehyde
CAS:1550-35-2 |
|
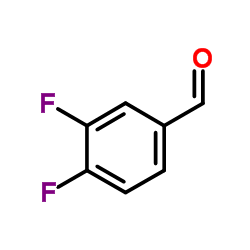 |
3,4-Difluorobenzaldehyde
CAS:34036-07-2 |
|
 |
4-Heptylbenzoyl chloride
CAS:50606-96-7 |
|
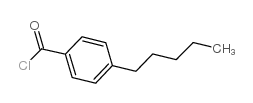 |
4-n-Pentylbenzoyl chloride
CAS:49763-65-7 |