An investigation of transient intermediates in the reaction of 2-methylglutamate with glutamate decarboxylase from Escherichia coli.
P L Grant, J M Basford, R A John
Index: Biochem. J. 241(3) , 699-704, (1987)
Full Text: HTML
Abstract
Several intermediates in the reaction of 2-methylglutamate with glutamate decarboxylase from Escherichia coli were detected by stopped-flow spectrophotometry and by rapid-scanning spectrophotometry after conventional mixing. Structures were assigned to intermediates on the basis of kinetic and spectral evidence. In the early stages of the reaction an intermediate with the properties expected of a geminal diamine accumulated significantly. Changes consistent with the conversion of this species into the external aldimine were also observed. The course of product formation was determined and linked with spectral changes taking place in the bound coenzyme. The effect of the minor decarboxylation-dependent transamination that accompanies the major reaction was analysed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
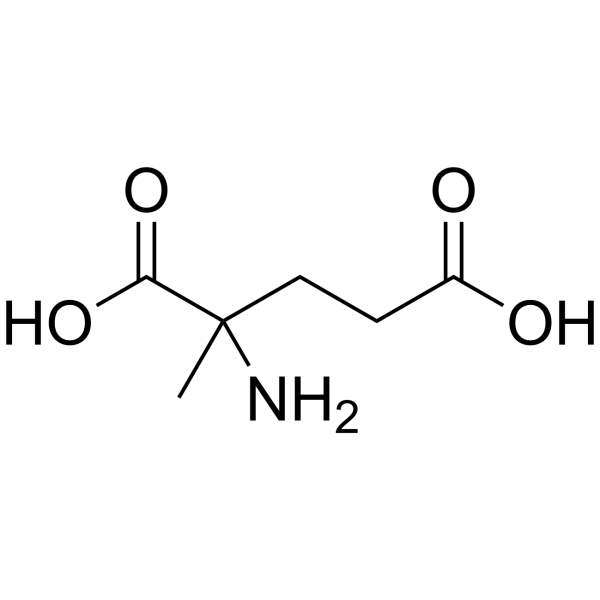 |
dl-2-methylglutamic acid
CAS:71-90-9 |
C6H11NO4 |
|
Characterization of 2-methylglyceric acid oligomers in secon...
2007-01-01 [J. Mass Spectrom. 42(1) , 101-16, (2007)] |
|
Crystal structure of phosphoserine aminotransferase from Esc...
1999-02-26 [J. Mol. Biol. 286(3) , 829-50, (1999)] |
|
Excitant activity of methyl derivatives of quinolinic acid o...
1984-01-01 [Br. J. Pharmacol. 81(1) , 175-81, (1984)] |
|
Characterization of the Escherichia coli K12 gltS glutamate ...
1991-03-01 [Mol. Gen. Genet. 225(3) , 379-86, (1991)] |
|
Effects of acidic amino acid antagonists upon the spectral p...
1985-11-01 [J. Neurosci. 5(11) , 2839-50, (1985)] |