| Structure | Name/CAS No. | Articles |
|---|---|---|
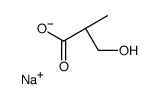 |
Sodium(R)-β-hydroxyisobutyrate
CAS:1228078-57-6 |
|
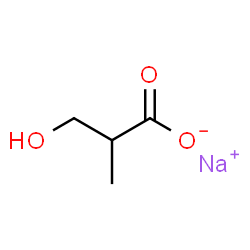 |
3-HIB
CAS:1219589-99-7 |