H2O2/UV-C treatment of textile preparation wastewater: kinetic investigation on alternative combinations of commercial textile preparation auxiliaries.
Idil Arslan-Alaton, Tugba Olmez-Hanci, Sarina Shayin
Index: Environ. Technol. 33(13-15) , 1531-7, (2012)
Full Text: HTML
Abstract
Four different textile preparation effluents were simulated to examine the applicability of the hydrogen peroxide/ultraviolet-C (H2O2/UV-C) advanced oxidation process for the treatment of real textile preparation (desizing, scouring and bleaching) wastewater bearing the non-ionic surfactant nonyl phenol decaethoxylate (NP-10). In the absence of any textile preparation chemical, NP-10 degradation was complete in 15 min (rate coefficient: 0.22 min(-1)) accompanied by 78% chemical oxygen demand (COD) (rate coefficient: 0.026 min(-1)) and 57% total organic carbon (TOC) (rate coefficient: 0.014 min(-1)) removals achieved after 60 min photochemical treatment. H2O2 consumption rates were not significantly affected by the introduction of carbonate and chloride ions (average rate coefficient: 0.032 min(-1)) at pH values <11.5, above which H2O2 dissociation to its conjugate base HO2(-) became pronounced. The organic, phosphonate-based sequestering agents competed with NP-10 for UV-C light absorption and HO* radicals. H2O2/UV-C oxidation of the simulated textile preparation effluent containing 3.0 g L(-1) Cl(-), 1.5 g L(-1) NaOH and 1.0 g L(-1) diethylenetriamine pentamethylene phosphonic acid (DTPMP) resulted in the worst treatment performance due to its high pH and organic carbon content. For this textile preparation effluent, NP-10 abatement was complete in 100min (rate coefficient: 0.018 min(-1)), while COD and TOC removals dropped down to only 16% and 8%, respectively, achieved after 60 min treatment. The highest H2O2/UV-C oxidation efficiency resulting in 34% COD and 28% TOC removals was obtained for the simulated textile preparation effluent comprising of 3.0 g L(-1) Cl(-), 1.5 g L(-1) NaOH and 1.0 g L(-1) 1-hydroxy ethylidene-1,1-diphosphonic acid (HEDP). For this textile preparation effluent, NP-10 degradation was complete after 50 min (rate coefficient: 0.061 min(-1)) exposure to H2O2/UV-C treatment.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
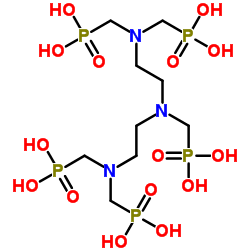 |
Diethylenetriaminepentakis(methylphosphonic acid)
CAS:15827-60-8 |
C9H28N3O15P5 |
|
Degradative hydrogen peroxide oxidation of chelates catalyse...
2003-05-20 [Sci. Total Environ. 307(1-3) , 11-8, (2003)] |
|
[Evaluation of 113mIn-DTPMP and 99mTc-MDP for bone scanning]...
1984-02-01 [Zhonghua Fang She Xue Za Zhi 18(1) , 58-62, (1984)] |
|
Determination of phosphonates in natural waters by ion-pair ...
1997-06-27 [J. Chromatogr. A. 773(1-2) , 139-46, (1997)] |
|
Thorium and actinium polyphosphonate compounds as bone-seeki...
2004-01-01 [Anticancer Res. 24 , 101-105, (2004)] |
|
The acute toxicity of gluconic acid, beta-alaninediacetic ac...
2003-04-01 [Arch. Environ. Contam. Toxicol. 44(3) , 332-5, (2003)] |