|
~81% |
|
~74% |
|
~89% |
|
~76% |

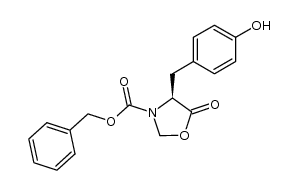
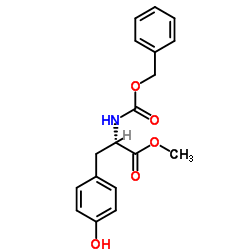
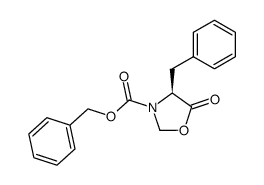


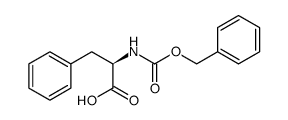
![Methyl N-[(benzyloxy)carbonyl]-L-methioninate Structure](https://image.chemsrc.com/caspic/311/56762-93-7.png)