Properties of a milk clotting protease isolated from fruits of Bromelia balansae Mez.
M F Pardo, L M López, N O Caffini, C L Natalucci
Index: Biol. Chem. 382 , 871-874, (2001)
Full Text: HTML
Abstract
Unripe fruit extracts of Bromelia balansae Mez (Bromeliaceae), whose principal endopeptidase is balansain I (isolated for anion exchange chromatography: pI = 5.45, molecular weight = 23192), exhibit a pH profile with a maximum activity around pH 9.0 and are inhibited only by cysteine peptidases inhibitors. The alanine and glutamine derivatives of N-alpha-carbobenzoxy-L-amino acid p-nitrophenyl esters were strongly preferred by the enzyme. Enzymatic hydrolysis of milk and soy proteins yield characteristic patterns at pH 9.0. The N-terminal sequence showed a very high homology (85-90%) with other known Bromeliaceae endopeptidases.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
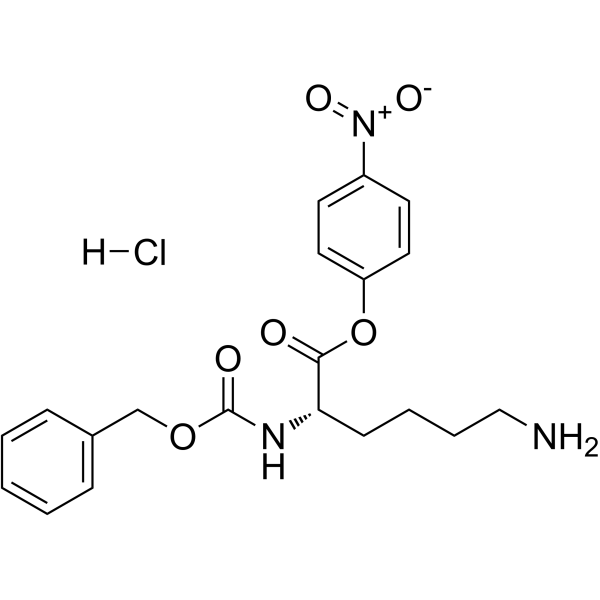 |
Z-Lys-ONp hydrochloride
CAS:2179-15-9 |
C20H24ClN3O6 |
|
[Inhibition of esterase by L-lysine, the activator and fibri...
1994-02-01 [Bioorg. Khim. 20(2) , 182-9, (1994)] |
|
Cryoenzymology of trypsin. A detailed kinetic study of the t...
1983-12-01 [Biochem. J. 215(3) , 555-63, (1983)] |
|
Effects of methanol cryosolvents on the structural and catal...
1986-01-25 [J. Biol. Chem. 261(3) , 1248-52, (1986)] |
|
The inactivation kinetics of papain by guanidine hydrochlori...
1998-11-01 [Biochim. Biophys. Acta 1388(1) , 84-92, (1998)] |
|
Purification of balansain I, an endopeptidase from unripe fr...
2000-09-01 [J. Agric. Food Chem. 48 , 3795-3800, (2000)] |