Synthesis of new fluorinated Tebufenpyrad analogs with acaricidal activity through regioselective pyrazole formation.
Santos Fustero, Raquel Román, Juan F Sanz-Cervera, Antonio Simón-Fuentes, Jorge Bueno, Salvador Villanova
Index: J. Org. Chem. 73(21) , 8545-52, (2008)
Full Text: HTML
Abstract
In previous studies, our group has shown that the use of fluorinated alcohols such as trifluoroethanol (TFE) and hexafluoroisopropanol (HFIP) as solvents dramatically increases the regioselectivity in the pyrazole formation from 1,3-diketone with methylhydrazine. We have now applied this synthetic method to the preparation of new fluorinated pyrazoles, which have then been used as synthetic intermediates in the preparation of fluorinated analogs of Tebufenpyrad, a commercial acaricide. These compounds display a strong acaricidal activity that is either comparable to or better than that of the commercial compound.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
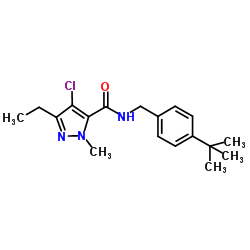 |
Tebufenpyrad
CAS:119168-77-3 |
C18H24ClN3O |
|
First detection of chlorfenapyr (Secure) resistance in two-s...
2003-01-01 [Exp. Appl. Acarol. 31(1-2) , 131-4, (2003)] |
|
New developments in insecticide resistance in the glasshouse...
2002-02-01 [Pest Manag. Sci. 58(2) , 123-30, (2002)] |
|
Ultraviolet radiation increases sensitivity to pesticides: s...
2011-09-01 [Bull. Environ. Contam. Toxicol. 87(3) , 231-7, (2011)] |
|
Incidence and inheritance of resistance to METI-acaricides i...
2001-05-01 [Pest Manag. Sci. 57(5) , 443-8, (2001)] |
|
[Acute tebufenpyrad and tolfenpyrad poisoning in humans].
2010-12-01 [Chudoku. Kenkyu. 23(4) , 324-8, (2010)] |