Improved regioselectivity in pyrazole formation through the use of fluorinated alcohols as solvents: synthesis and biological activity of fluorinated tebufenpyrad analogs.
Santos Fustero, Raquel Román, Juan F Sanz-Cervera, Antonio Simón-Fuentes, Ana C Cuñat, Salvador Villanova, Marcelo Murguía
Index: J. Org. Chem. 73(9) , 3523-9, (2008)
Full Text: HTML
Abstract
The preparation of N-methylpyrazoles is usually accomplished through reaction of a suitable 1,3-diketone with methylhydrazine in ethanol as the solvent. This strategy, however, leads to the formation of regioisomeric mixtures of N-methylpyrazoles, which sometimes are difficult to separate. We have determined that the use of fluorinated alcohols such as 2,2,2-trifluoroethanol (TFE) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as solvents dramatically increases the regioselectivity in the pyrazole formation, and we have used this modification in a straightforward synthesis of fluorinated analogs of Tebufenpyrad with acaricide activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
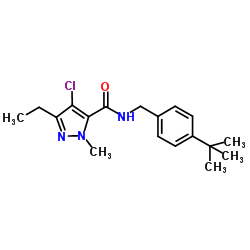 |
Tebufenpyrad
CAS:119168-77-3 |
C18H24ClN3O |
|
First detection of chlorfenapyr (Secure) resistance in two-s...
2003-01-01 [Exp. Appl. Acarol. 31(1-2) , 131-4, (2003)] |
|
New developments in insecticide resistance in the glasshouse...
2002-02-01 [Pest Manag. Sci. 58(2) , 123-30, (2002)] |
|
Ultraviolet radiation increases sensitivity to pesticides: s...
2011-09-01 [Bull. Environ. Contam. Toxicol. 87(3) , 231-7, (2011)] |
|
Incidence and inheritance of resistance to METI-acaricides i...
2001-05-01 [Pest Manag. Sci. 57(5) , 443-8, (2001)] |
|
[Acute tebufenpyrad and tolfenpyrad poisoning in humans].
2010-12-01 [Chudoku. Kenkyu. 23(4) , 324-8, (2010)] |