Simultaneous determination of liensinine, isoliensinine and neferine from seed embryo of Nelumbo nucifera Gaertn. in rat plasma by a rapid HPLC method and its application to a pharmacokinetic study.
Ying Huang, Libo Zhao, Yun Bai, Ping Liu, Jialing Wang, Jizhou Xiang
Index: Arzneimittelforschung 61(6) , 347-52, (2011)
Full Text: HTML
Abstract
A rapid and specific HPLC method has been developed and validated for the simultaneous determination of liensinine (CAS 2586-96-1), isoliensinine (CAS 6817-41-0) and neferine (CAS 2292-16-2) in rat plasma. The sample was prepared by a liquid-liquid extraction with diethyl ether and the recovery was above 80% from the plasma for the three compounds. Chromatographic separation was achieved with a Hypersil BDS C18 column (4.0 mm x 250 mm, particle size 5 microm). A mobile phase consisting of methanol: 0.2 M KH2PO4:0.2 M NaOH:triethylamine (71:17:12:0.002, v/v/v/v, pH 9.2-9.3) was slowly delivered at 0.8 ml/min in isocratic mode with a detection wavelength of 282 nm. The linearity of calibration curves were good (r > 0.999) in the concentration range of 0.031-2.00 microg/ ml. The lower limit of quantification can reach 0.03 microg/ml for the three compounds. The intra-day and inter-day variations estimated with QC samples were less than 8% for the three tested concentration levels. This developed method was applied in the plasma pharmacokinetic study of total bisbenzylisoquinoline alkaloids (TAL) of the lotus flower (Lian Zi Xin) following a single oral and intravenous administration of TAL in rats.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
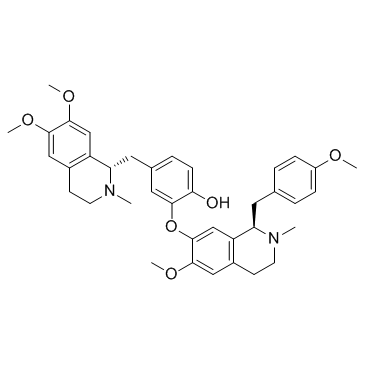 |
Neferine
CAS:2292-16-2 |
C38H44N2O6 |
|
In vitro opioid receptor affinity and in vivo behavioral stu...
2015-11-04 [J. Ethnopharmacol. 174 , 57-65, (2015)] |
|
Anti-amnesic activity of neferine with antioxidant and anti-...
2010-09-25 [Life Sci. 87(13-14) , 420-30, (2010)] |
|
Blockade of HERG K+ channel by isoquinoline alkaloid neferin...
2009-08-01 [Naunyn Schmiedebergs Arch. Pharmacol. 380(2) , 143-51, (2009)] |
|
Synthesis and pharmacological activity of alkaloids from emb...
2013-01-01 [Chem. Pharm. Bull. 61(1) , 59-68, (2013)] |
|
Effect of neferine on liver ischemia-reperfusion injury in r...
2011-09-01 [Transplant. Proc. 43(7) , 2536-9, (2011)] |