Triamino derivatives of triazolotriazine and triazolopyrimidine as adenosine A2a receptor antagonists.
Chi B Vu, Pamela Shields, Bo Peng, Gnanasambandam Kumaravel, Xiaowei Jin, Deepali Phadke, Joy Wang, Thomas Engber, Eman Ayyub, Russell C Petter
Index: Bioorg. Med. Chem. Lett. 14(19) , 4835-8, (2004)
Full Text: HTML
Abstract
Piperazine derivatives of 2-furanyl[1,2,4]triazolo[1,5-a][1,3,5]triazine have recently been shown to be potent and selective adenosine A(2a) receptor antagonists. We now demonstrate that potent and selective A(2a) receptor antagonists could still be obtained when the arylpiperazines are separated from the triazolotriazine core structure by an ethylenediamine spacer. Selected analogs bearing this triazolotriazine or the related triazolopyrimidine core structure have been found to be orally active in a mouse catalepsy model of Parkinson's disease.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
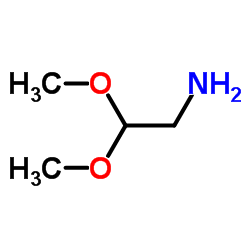 |
2,2-Dimethoxyethanamine
CAS:22483-09-6 |
C4H11NO2 |
|
Altering in vivo macrophage responses with modified polymer ...
2015-07-01 [Biomaterials 56 , 187-97, (2015)] |
|
Effect of surface modification and macrophage phenotype on p...
2014-11-10 [Biomacromolecules 15(11) , 4102-10, (2014)] |
|
An extremely efficient three-component reaction of aldehydes...
2007-02-16 [J. Org. Chem. 72(4) , 1263, (2007)] |
|
Isolation of primitive human hematopoietic progenitors on th...
1999-08-03 [Proc. Natl. Acad. Sci. U. S. A. 96(16) , 9118-23, (1999)] |
|
Synthesis of a chitosan-dendrimer hybrid and its biodegradat...
2003-01-01 [Biomacromolecules 4(5) , 1244-9, (2003)] |