Adenosine cyclic 3',5',-monophosphate phosphodiesterasr inhibitors. 2.3-Substituted 5,7-dialkylpyrazolo [1,5-a]pyrimidines.
T Novinson, J P Miller, M Scholten, R K Robins, L N Simon, D E O'Brien, R B Meyer
Index: J. Med. Chem. 18(5) , 460-4, (1975)
Full Text: HTML
Abstract
A number of 3-bromo-, 3-nitro-, and 3-ethoxycarbonyl-5,7-dialkylpyrazolo[1,5-a]pyrimidines were synthesized and screened as in vitro cAMP phosphodiesterase inhibitors. The condensation of 3-aminopyrazole with symmetrical beta-diketones (acetylacetone, heptane-3,5-dione, etc.) afforded symmetrical dialkylpyrazolo[1,5-a]pyrimidines (5). The reaction of 3-aminopyrazole with unsymmetrical beta-diketones (hexane-2,4-dione, heptane-3,5-dione, etc.) gave a mixture of 5-methyl-7-alkylpyrazolo[1,5-a]pyrimidine (3) and 5-alkyl-7-methylpyrazolo[1,5-a]pyrimidines (4). The technique for the separation of 3 from 4 is described. The inhibition constants, alpha (the ratio of the molar I50 of theophylline to the molar I50 of the test compounds), were subjected to a Hansch correlation analysis. The results indicated that PDE isolated from beef heart tissue was most sensitive to changes in the length of the alkyl group in the 5 position of the pyrazolo[1,5-a]pyrimidine ring, whereas the PDE isolated from rabbit lung tissue was more sensitive to changes in the length of the 7-alkyl group. Experimentally and theoretically, the n-propyl group was found to approximate the ideal size for the alkyl group in both the 5 and 7 positions;5,7-di-n-propyl-3-ethoxycarbonylpyrazolo[1,5-a]pyrimidine (5e) was the most potent inhibitor of both lung and heart PDE.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
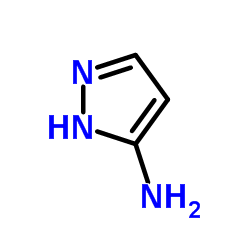 |
3-Aminopyrazole
CAS:1820-80-0 |
C3H5N3 |
|
Trypanotoxic activity of thiosemicarbazone iron chelators.
2015-03-01 [Exp. Parasitol. 150 , 7-12, (2015)] |
|
Vacuolar-ATPase Inhibition Blocks Iron Metabolism to Mediate...
2015-07-15 [Cancer Res. 75 , 2863-74, (2015)] |
|
Analysis of gait in rats with olivocerebellar lesions and ab...
2015-09-15 [Behav. Brain Res. 291 , 342-50, (2015)] |
|
Identification of ribonucleotide reductase M2 as a potential...
2014-01-01 [Cell Cycle 13(2) , 199-207, (2014)] |
|
[Trends Heterocycl. Chem. 2 , 97, (1991)] |